Explain the Differences Between Weak-field and Strong-field Metal Complexes
A complex can be classified as high spin or low spin. Strong field metal complexes means complees with low spin ligands and weak field omplexes means complexes with high spin ligands.

Crystal Field Theory An Overview Sciencedirect Topics
Examples include chloride ions fluoride ions etc.
. Weve got the study and writing resources you need for your assignments. This E-mail is already registered as a. Solved Expert Answer to Explain the differences between weak-field and strong-field metal complexes.
Start your trial now. They are also called high spin complexes. A Molecular Approach 4th Edition 4th Edition Nivaldo J.
When talking about all the molecular geometries we compare the crystal field splitting energy ΔΔ View the full answer. Spectrochemical series gives the arrangement of ligands in the increasing order of crystal field splitting. Explain the difference between weak-field and strong-field metal complexes.
First week only 499. This problem has been solved. They are formed when the crystal field stabilisation energy Δ 0 is greater than the p.
Explain the difference between weak-field and strong-field metal complexes. When we compare the energy of splitting with respective to electrostatic force between electrons the occupancy and no of unpaired electrons can be estimated. We have step-by-step solutions for.
Its when we talk about strong field. Explain the differences between weak-field and strong-field metal complexes. Hello everyone this is Ricky and they were working on Problem number 11 from Chapter 22.
They form low spin complexes. Still field splitting energy his energy that is released when the D or well split. Key Difference Strong Ligand vs Weak Ligand.
A ligand is an atom ion or a molecule that donates or shares two of its electrons through a coordinate covalent bond with a central atom or ion. Explain why compounds of Zn2 are white but compounds of Cu2 are often blue or green. Experts are tested by Chegg as specialists in their subject area.
The ligand which causes large spliting of the d orbitals ie. High spin and low spin are two possible classifications of spin states that oc View the full answer. Please type your answer.
Around 200 words please. We review their content and use your feedback to keep the quality high. Large Δ o are called strong field.
The difference in energy between the split d-orbittals in the presence of ligands is called crystal. Explain why compounds of Sc3 are colorless but compounds of Ti3 are colored. High spin complexes are weak field complexes and low spin complexes are strong field complexes.
Um so first lets introduce crystal Feel theory. 1They are formed when the crystal field stabilisation energy Δ 0 in octahedral complexes is less than the energy required for an electron pairing in a single orbital p. The magnitude of splitting compared to the electron -electron repulsions determines the d orbital occupancy and therefore the number of unpaired electrons.
Start your trial now. 3 rows Weak field and strong field ligand. Weak field ligands induce less splitting of the crystal.
Field splitting energy Δ o. The concept of ligands is discussed under coordination chemistry. Explain the differences between weak-field and strong-field metal complexes.
Who are the experts. Please type your answer. Explain the difference between weak-field and strong-field metal complexes.
Textbook solution for Chemistry. Up to 256 cash back Get the detailed answer. Um to help us understand um bonding and also help explain the colors and magnetic.
Were thinking about large splitting of purples a large amount of energy when we talk about weak field slogans. Strong field ligands cause greater crystal field splitting. Ligands are chemical species that are involved in the formation of complexes with metal ions.
Explain the difference between weak-field and strong-field metal complexes. Tro Chapter 25 Problem 11E. Strong Field transition metals have a large crystal field splitting energy and the weak field transition metals have a small crystal field splitting energy.
Ill be explaining the difference between weak field and strong fields medal complexes. Explain the difference between weak-field and strong-field metal complexes. Expert solutions for Question Explain the difference between weak-field and strong-field metal complexes.
Weve got the study and writing resources you need for your assignments. This could be calculated by comparing this put energy uh to the electrostatic forces between the electrons in a period of time period electrons. Solution for Explain the differences between weak-field and strong-field metal complexes.
Ligands with large splitting of d orbitals are called strong field ligands and ligands wich causes small crystal field splitting are called weak field ligands. Crystal field theory is a model for transition metal complexes. They form high spin complexes.
Solution for Explain the differences between weak-field and strong-fieldmetal complexes. First week only 499. Science Chemistry QA Library Explain the.
Weak field ligands cause less crystal field splitting.
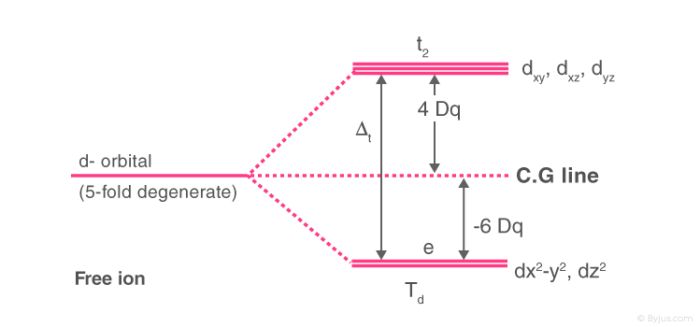
Crystal Field Theory Cft Detailed Explanation With Examples Videos
5 4 Spectrochemical Series Chemistry Libretexts
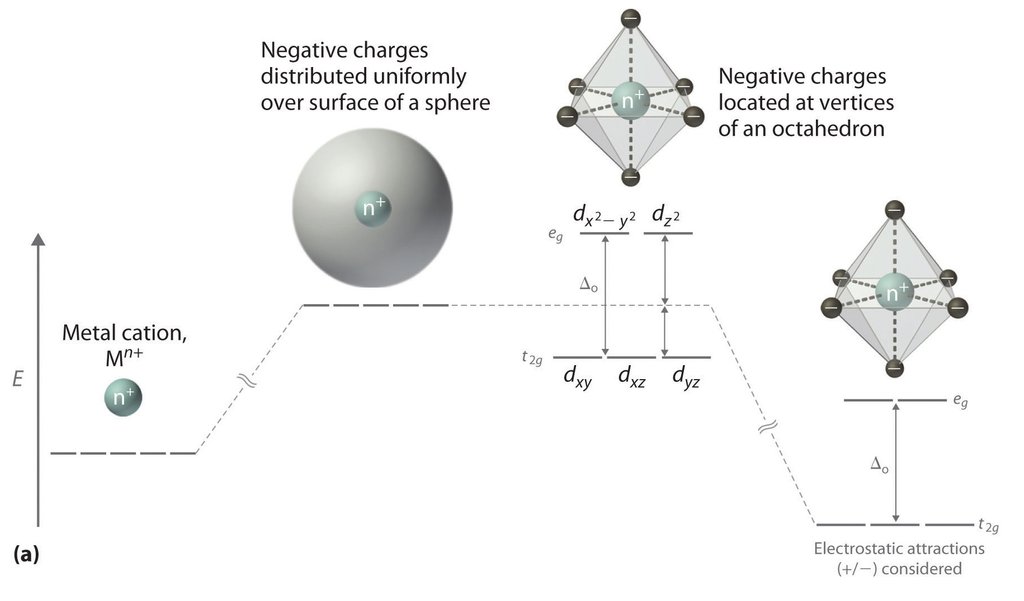
Crystal Field Theory Definition Examples Diagrams

Crystal Field Theory Definition Examples Diagrams

Ligand Field Theory An Overview Sciencedirect Topics

Strong And Weak Field Ligands Coordination Chemistry Youtube

Strong And Weak Field Ligands Coordination Chemistry Youtube
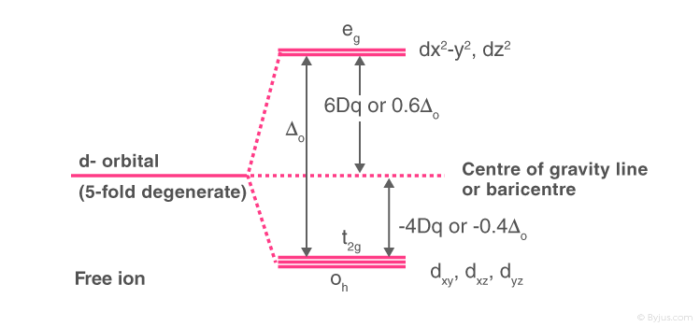
Crystal Field Theory Cft Detailed Explanation With Examples Videos

Ligand Field Theory An Overview Sciencedirect Topics

Strong And Weak Field Ligands Coordination Chemistry Youtube

Spectrochemical Series Definition Classes Of Ligands Video Lesson Transcript Study Com

A New Theory Of Magnetar Formation Neutron Star Theories Neutrons
Is An Oxalate Ion A Strong Field Ligand Quora

Coordination Compound Ligand Field And Molecular Orbital Theories Britannica
5 4 Spectrochemical Series Chemistry Libretexts

What Is Spectrochemical Series Explain The Difference Between A Weak Field Ligand And A Strong Field Ligand

Spectrochemical Series Definition Classes Of Ligands Video Lesson Transcript Study Com


Comments
Post a Comment